Wearble Medical Device Design & Engineering
Wearble Medical Device Design & Engineering
We specialize in end-to-end development of connected and wearable medical devices, combining expertise in electronics, software, and regulatory compliance to help clients move faster from concept to market.
Our Services
From product ideation to post-market support, ITR provides a full range of MedTech engineering services aligned with ISO 13485 and IEC 62304 standards.
Our Expertise
Our multidisciplinary teams integrate hardware, software, and data science to deliver reliable, compliant, and commercially viable MedTech solutions.
Designs that balance form, function, and user experience so your product is not only reliable but also intuitive to use.
From PCB design to mechanical housing, we build the core hardware systems that keep your device safe, scalable, and production-ready.
Embedded systems and applications that run smoothly, integrate securely, and meet the performance demands of medical devices.
Cloud platforms and infrastructure built for scale enabling secure data handling, AI-driven insights, and efficient deployments.
Applied research to bring AI/ML to real-world devices, from edge computing to large-scale cloud processing.
Design controls, documentation, and compliance strategies that align with ISO 13485, IEC 62304, FDA, and CE requirements.
Explore ITR’s MedTech R&D Platforms
Over the years, ITR has built multiple hardware and algorithmic platforms to help partners validate ideas, collect biosignals, and accelerate prototyping.
Each platform is validated, customizable, and supported by our engineering expertise to advance your project from research to development.

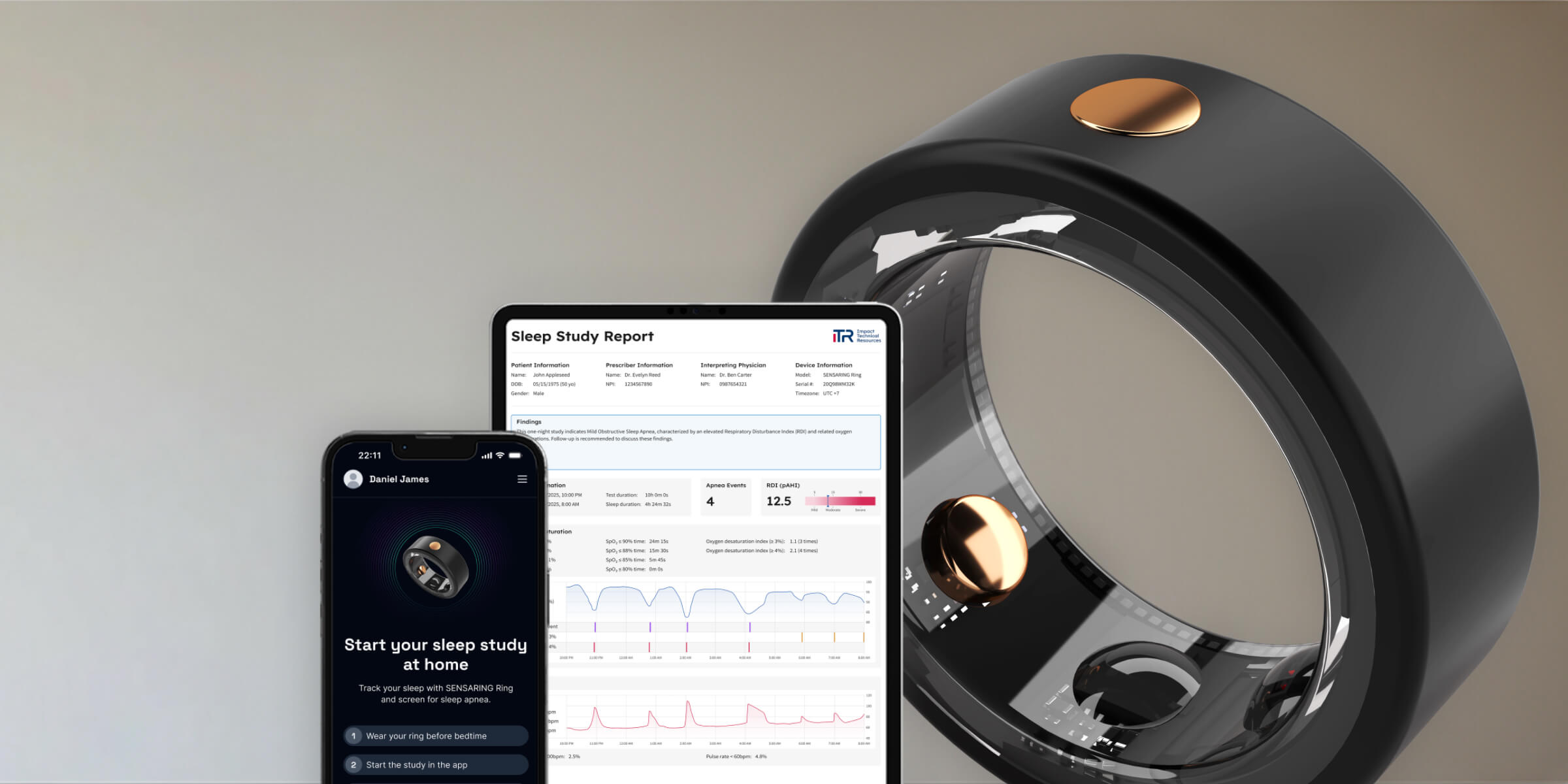
A medical-grade biosensor ring platform for tracking multi-modal physiological data. Ideal for algorithm validation and device prototyping.


A skin temperature sensing module with SDK, enabling thermal tracking for wearable or clinical applications.


A wrist-mounted biosensing platform designed for multi-modal signal acquisition, including PPG, EDA, and motion data. Built for flexible integration and rapid algorithm testing in MedTech R&D projects.

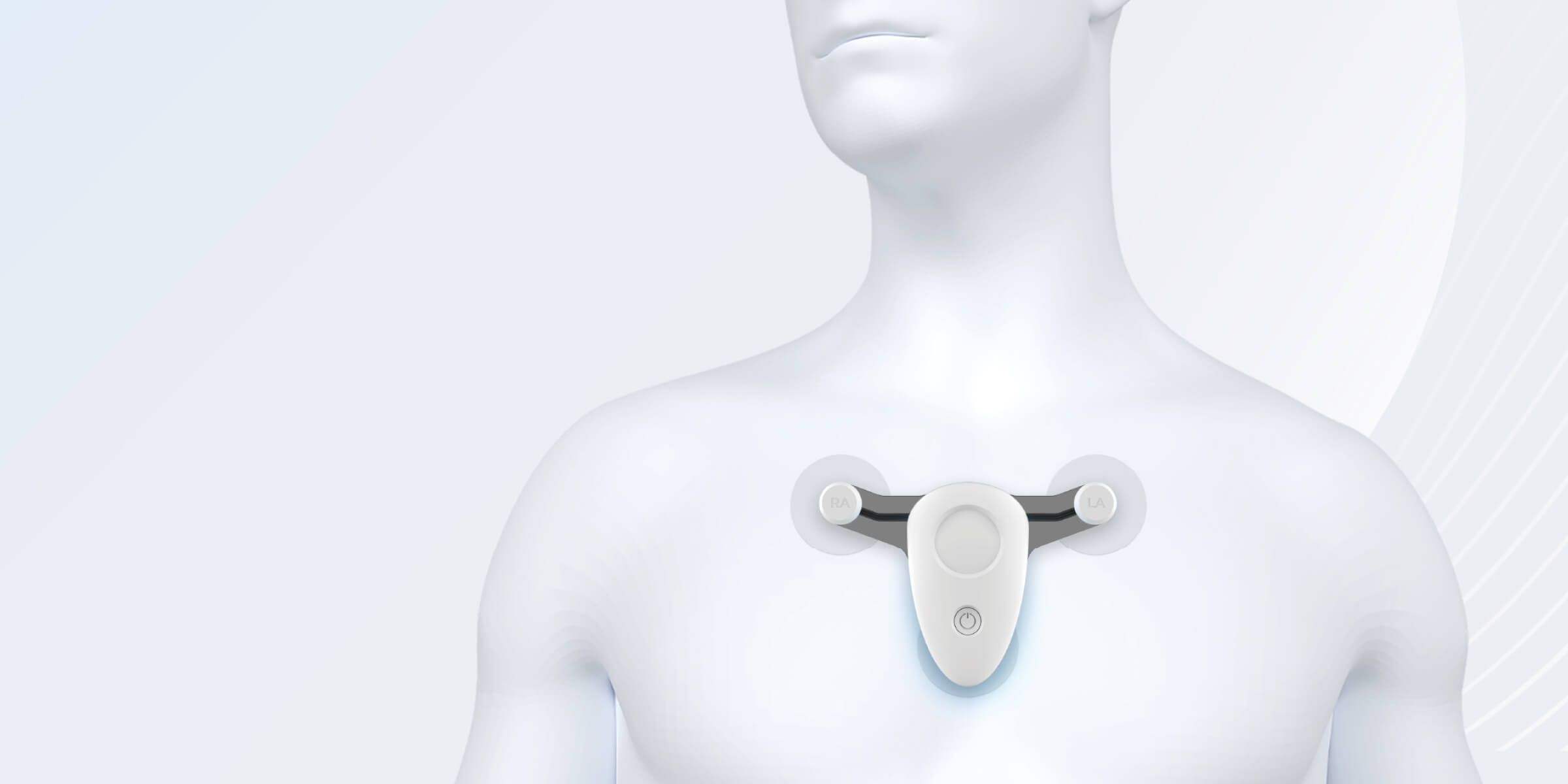
A compact, high-fidelity ECG recorder for cardiac monitoring and signal algorithm testing.


A full system for sleep tracking & respiratory analysis. Supports modular sensors for HR, SpO2, airflow, and body position.


A connected care platform integrating multiple biosensors for remote patient monitoring and chronic condition management.
Interested in our R&D platforms? Submit the form to receive instant access to comprehensive documentation and guides.

Trust & Proof
























Contact & Inquiry
Let’s Discuss Your MedTech Project
Whether you’re exploring early-stage concepts, looking for a reliable engineering partner, or planning your next clinical-ready device - we’d love to hear from you.
Visit our main website to explore our engineering capabilities, services, and recent innovations in MedTech.











