Medtech Webinar
Funding Is Where Most MedTech Startups Stall
Funding Is Where Most MedTech Startups Stall
Founders often build their medical devices in the early stage using personal capital or loans, but without a clear funding strategy, investors won’t invest. Curious about what investors want?

Medtech Failures
Are you sure your startup is using its capital the right way?
Many founders run out of cash before fundraising because they fail to balance product development with commercialization. No revenue no runway. Shut down.

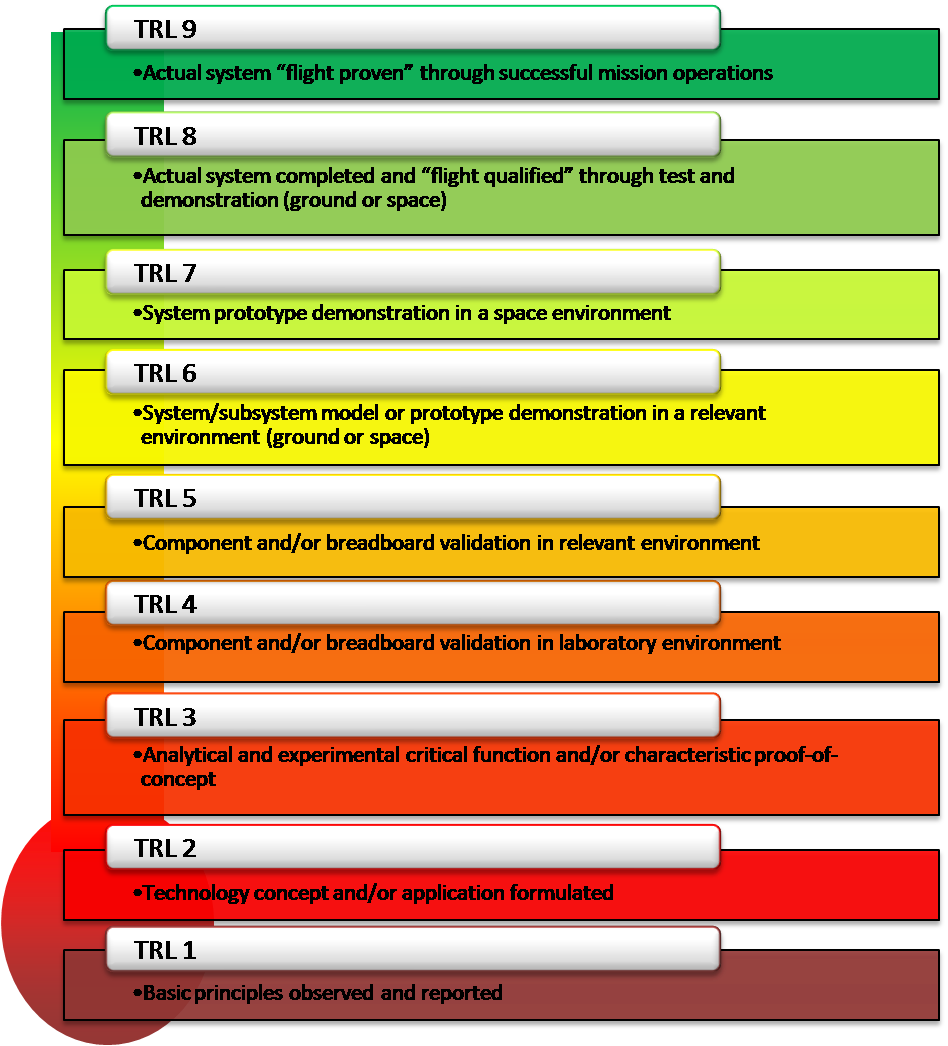
What is TRL?
NASA-Verified Definition of Technical Readiness Levels (TRL)
Technology Readiness Levels (TRL) are a type of measurement system used to assess the maturity level of a particular technology.
Each technology project is evaluated against the parameters for each technology level and is then assigned a TRL rating based on the projects progress.
There are nine technology readiness levels. TRL 1 is the lowest and TRL 9 is the highest.
Who we are?
We are ITR.
A technical partner built for MedTech.
We help MedTech startups turn concepts into clinically validated, investor-ready products.
What can MedTech founders actually gain from this event?
Event Agenda
Explore the Funding Readiness for MedTech Startups agenda
Streaming live on Microsoft Teams - July 25, 2025
14:00 (UTC +1 - Berlin/Madrid) · 19:00 (UTC +7 - Vietnam)
Meet Our Speakers
What Funding Readiness Really Means in MedTech








Register Now
Join our Free Exclusive MedTech Event packed with insights and built for global impact
Special Offer

Explore MOre InSights
Strategic insight meets real-world impact in Medtech
From funding frameworks to international partnerships, we’re here to support your innovative Medtech products.












.jpg)
