
Bringing a medical wearable from concept to validation has never been easy. Teams may design impressive prototypes, yet months later still struggle to produce the one thing that truly matters: reliable biosignal data.
In the MedTech space, validation is where innovation meets evidence. It’s also where many startups lose momentum not because their idea is weak, but because the path to collecting compliant, high-quality data is longer and more complex than expected.
Some teams try to build their own biosensors to control data quality. Others use open-source kits, license datasets, or collaborate with hospitals. Each approach works to a degree but they all face the same trade-off between time, control, and regulatory compliance.
This article examines why these bottlenecks persist across the industry and how platform-based biosensor systems such as SensaRing are reshaping the way MedTech teams validate algorithms.
1. The Validation Bottleneck: More Than a Technical Problem
On paper, validation looks straightforward: collect data, test your model, publish results. In practice, it’s a balancing act between hardware readiness, data accessibility, and documentation.
Even for experienced engineering teams, these three components often move at different speeds:
- Hardware development follows manufacturing timelines.
- Data collection depends on stable firmware and study logistics.
- Regulatory documentation requires structured evidence from the start.
When these processes don’t align, projects stall. The delay isn’t caused by lack of expertise it’s the fragmented nature of MedTech product development.
2. The Hardware Dilemma: Build, Buy, or Borrow
Early-stage MedTech teams face a fundamental choice: build a custom device, use an existing kit, or partner with a manufacturer. Each path has strengths and hidden costs.
- Building from scratch provides control but takes 12–18 months before the first usable prototype. You need electrical engineers, firmware developers, QA, and test labs often before any data exists to validate your core algorithm.
- Using open-source or research kits (Shimmer, BITalino, or Empatica) speeds up experimentation but rarely meets ISO or FDA-grade documentation needs.
- Partnering with an ODM/OEM can reduce workload, but limits access to firmware and raw data, locking teams into vendor-dependent ecosystems. This is a common challenge, which is why many Medical OEM leaders are now leveraging Independent Design Houses (IDHs) for product development to gain more flexibility
In other words, there’s no easy choice. Each option solves one constraint while introducing another usually cost, time, or compliance.
That’s why many innovators now look to platform-based biosensors: devices built under medical standards but offered as configurable research tools. They compress the hardware timeline without sacrificing data quality.
3. The Data Access and Collection Challenge
Even when hardware exists, collecting usable biosignal data is another challenge altogether. Commercial wearables often restrict access to raw signals, providing only derived metrics like heart rate or SpO₂ averages. For machine learning or digital biomarker projects, these summaries aren’t enough.
To build clinically valid algorithms, researchers need:
- Raw waveforms (e.g., PPG, acceleration, temperature)
- High sampling rates and minimal filtering
- Consistent calibration and metadata control
Some teams try to compensate by buying datasets or collaborating with hospitals. While this offers a shortcut, it comes with its own drawbacks: limited sensor configurations, heterogeneous data formats, and uncertainty about collection protocols.
Accessing your own raw biosignals, in contrast, enables reproducibility, reprocessing, and traceability - all critical in clinical validation.
4. Regulatory Documentation: The Hidden Layer of Validation
Every dataset used for clinical validation must come from a process that aligns with ISO 13485 (Quality Management) and IEC 62304 (Software Lifecycle Management). This doesn’t just mean that the final product must comply; the data acquisition tools and the development workflow also need traceable documentation.
For small teams, this often becomes an unexpected cost center. Hiring a regulatory consultant helps, but preparing design history files, risk assessments, and version-controlled test records still requires weeks of structured documentation.
A common scenario:
A startup completes a proof-of-concept study, only to discover that their data is not admissible for regulatory submissions because the device used wasn’t developed under compliant conditions. That means starting the entire validation cycle again from zero.
Platforms like SensaRing remove this uncertainty by ensuring that the hardware and software already follow ISO and IEC processes. This doesn’t eliminate the need for documentation, but it anchors validation on a compliant foundation, saving months of rework later.
5. How the SENSARING Platform Simplifies the Journey
The SensaRing Platform represents a practical middle ground between full in-house development and off-the-shelf kits. It offers a ready-to-use biosensor system designed with compliance, data access, and customization in mind.
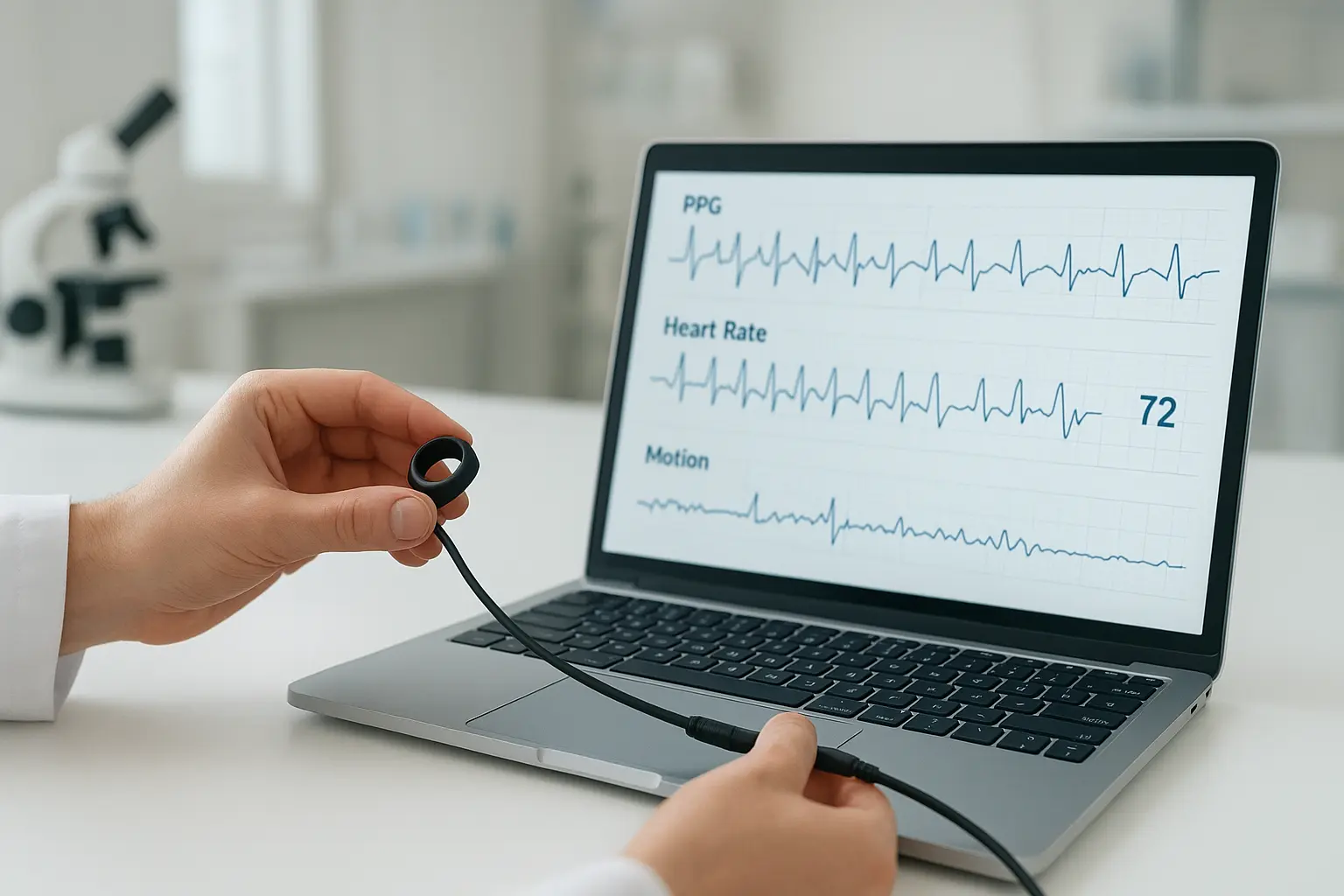
- Medical-grade hardware: PPG, SpO₂, motion, and temperature sensors in a compact ring form factor.
- Full raw data access: SDK and API for direct streaming and export, enabling precise signal processing.
- Regulatory-aligned design: Developed under ISO 13485 and IEC 62304 to support future FDA/MDR submissions.
- Fast onboarding: Data collection can begin within weeks, not months.
Instead of reinventing hardware, teams can focus on algorithm development, digital biomarker discovery, and data analysis - the core of their innovation.

6. Practical Scenarios Where SensaRing Accelerates Validation
- Sleep and Stress Analysis
A European research group used SensaRing to collect continuous PPG and motion signals to refine their sleep-stage classification model. The team reached clinical-grade accuracy within six weeks a timeline typically requiring several months with custom hardware. - Cardiovascular Monitoring
A U.S.-based startup validated its arrhythmia detection algorithm using raw PPG from SENSARING, achieving 15% higher signal correlation versus consumer wearables. - Digital Biomarker Exploration
A South Korean academic lab used SensaRing for HRV and fatigue detection studies, reducing data-collection setup time from four months to one.
The cardiovascular monitoring case study above is a practical example of a much larger trend. For a comprehensive look at how these devices are changing the field, explore our in-depth article: Wearable Tech Redefining Cardiovascular Disease Management: What You Need to Know
These cases show a consistent trend: platform-based validation shortens the experimental loop while maintaining traceable data integrity.
7. Comparative Impact: Traditional vs. Platform-Based Validation
8. From Proof to Evidence
Validation is not just a milestone it’s a maturity test for every MedTech innovation. It determines whether an idea can withstand regulatory scrutiny and clinical expectations.
While multiple paths exist from open-source kits to OEM partnerships the underlying goal remains the same: generate trustworthy biosignal data quickly, reproducibly, and under compliant conditions.
That’s where SENSARING provides leverage. It doesn’t replace the engineering process but accelerates the stages that matter most to scientific validation letting teams move from concept to clinical proof without rebuilding the fundamentals.
In MedTech, success isn’t measured by how many prototypes you build - but by how quickly you can prove one work





