
Introduction
Over the past decade, AI in MedTech and medical wearables have transformed how health data is collected and interpreted. Devices today track heart rate, sleep stages, oxygen saturation, and stress levels. Yet despite rapid development, many of these innovations remain trapped at the prototype stage.
The reason isn’t a lack of engineering talent it’s the absence of clinically validated biosignal data. Access to raw biosignals unfiltered physiological signals captured directly from sensors is what determines whether an idea becomes a regulated, trustworthy medical product.
Without access to raw biosignal data, innovation remains theoretical.
What Are Raw Biosignals?
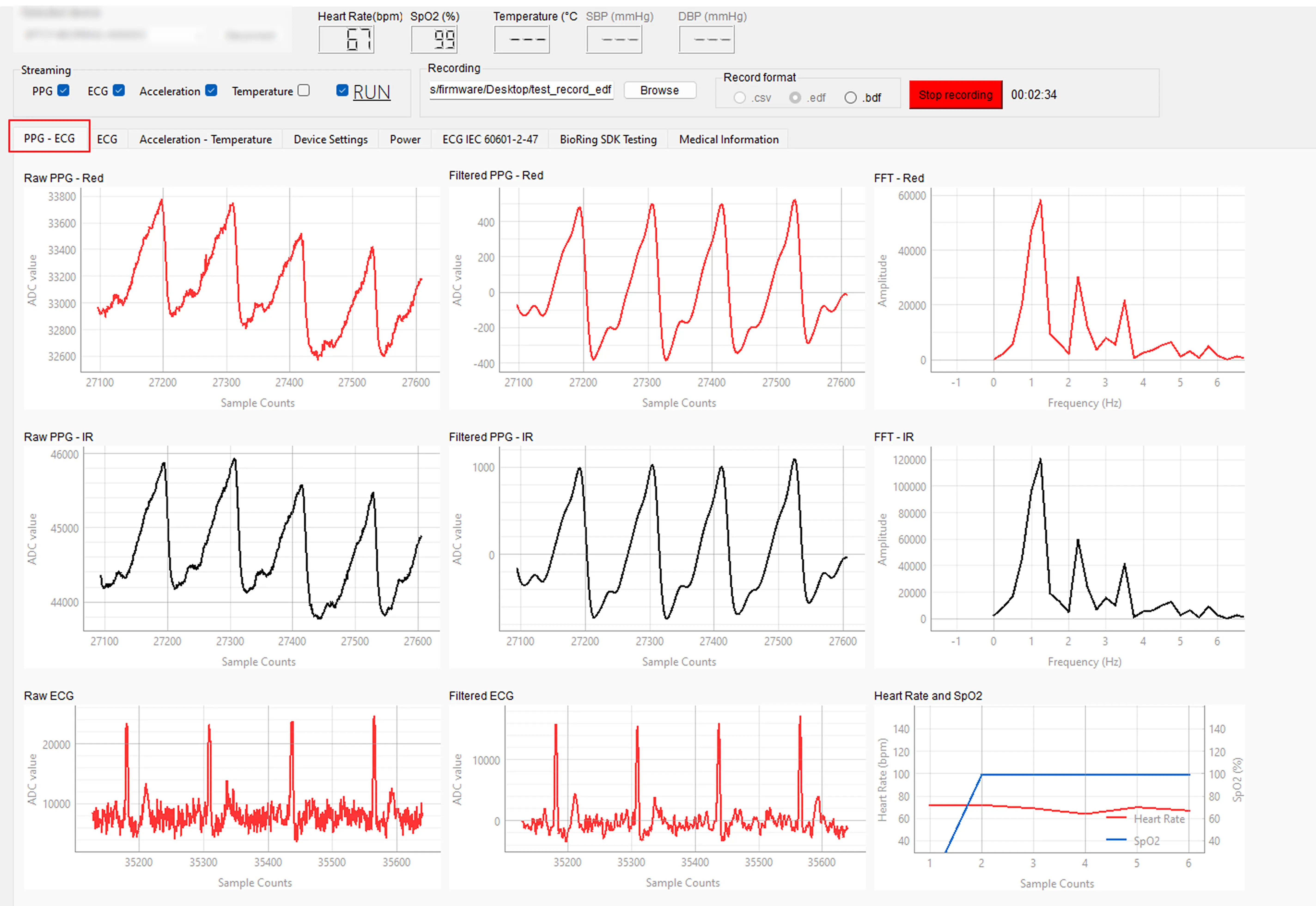
A biosignal is any measurable indicator of biological activity—heartbeats, respiration, body movement, or temperature fluctuations. Raw biosignals refer to the unprocessed waveforms captured directly by sensors such as:
- PPG (Photoplethysmography)
- ECG (Electrocardiogram)
- Accelerometer and temperature sensors
In contrast, processed data (like average heart rate or step count) compresses and loses valuable details. Raw data preserves the entire waveform, which is essential for:
- Developing and training AI models
- Detecting subtle physiological changes
- Conducting reproducible, auditable clinical studies
Example: a smartwatch may report a heart rate of 65 bpm, but to compute heart rate variability (HRV) or stress response, researchers need millisecond-level raw PPG signals.
Why Access Is So Limited
Most MedTech teams face restricted access to raw biosignal data. Commercial wearables (e.g., Apple Watch, Fitbit, Garmin) are closed systems—designed for user experience, not R&D. Their APIs expose only processed values due to:
- Proprietary firmware
- Privacy constraints
- Limited hardware bandwidth
This leads to three major issues:
- AI model limitations: Algorithms trained on averaged metrics miss critical signal variability.
- Inadequate data resolution: Consumer-grade sampling rates are unsuitable for clinical validation.
- Extended R&D cycles: Startups often spend 12–18 months building prototype hardware just to acquire usable raw data.
Innovation in digital health slows not from lack of ideas, but from lack of open biosignal access.
How Raw Biosignals Bridge Innovation and Clinical Validation
Access to raw biosignal data closes the gap between concept and clinical evidence through three key mechanisms:
1. Accelerating AI Model Development
Training deep learning algorithms on raw signals enables:
- Identification of novel digital biomarkers
- Detection of arrhythmias, stress, and sleep patterns
- Optimization of models for specific populations or diseases
2. Ensuring Medical-Grade Reliability
In medical AI, reproducibility and transparency are non-negotiable.
Raw data supports:
- Algorithm auditability for CE-MDR and FDA submissions
- Validation of signal integrity across studies
3. Fostering Collaborative R&D
Shared access to high-quality raw datasets allows:
- Faster prototyping across labs and OEMs
- Reduced duplication in sensor and SDK development
- Greater focus on insight generation rather than device fabrication
Real-World Use Cases
Examples of research enabled by raw biosignals include:
- Sleep analysis: Classifying sleep stages from PPG and accelerometer data without EEG setups.
- Cardiac monitoring: Detecting early arrhythmia via PPG waveform variations.
- Stress and emotion tracking: Using HRV and EDA data from ring sensors to quantify stress levels.
- Cuffless blood pressure monitoring: Estimating continuous BP from PPG waveform morphology.
These applications highlight how raw biosignals turn digital prototypes into clinically validated medical tools.
The SENSARING Platform: A Technical Enabler for Biosignal Research
To overcome the accessibility barrier, ITR designed the SENSARING Platform a development ecosystem for researchers and MedTech startups focused on biosignal-based innovation.

Unlike consumer devices, SensaRing is engineered for transparency and data integrity.
Technical Highlights:
- Multi-sensor ring hardware: PPG, SpO₂, accelerometer, temperature, optional ECG
- Raw data SDK and API: Full, timestamped biosignal stream access
- Regulatory-aligned framework: Built under ISO 13485 and IEC 62304 principles
By shortening data acquisition from months to weeks, SensaRing allows R&D teams to validate algorithms, conduct pilot studies, and accelerate clinical readiness without reinventing the hardware layer.

Conclusion
Raw biosignals are the foundation of evidence-based innovation in digital health.
They provide the precision, traceability, and reproducibility required to translate algorithms into medical-grade technologies.
As the MedTech industry advances toward AI-driven care, open access to raw biosignal data will define who leads the next generation of healthcare breakthroughs.
Platforms like SENSARing exemplify how open, standards-driven development can transform innovation into measurable clinical impact.
#rawbiosignals #biosignaldata #MedTechR&D #AIinMedTech #digitalhealth #medicalwearables #clinicalvalidation #SensaRing #digitalbiomarkers





