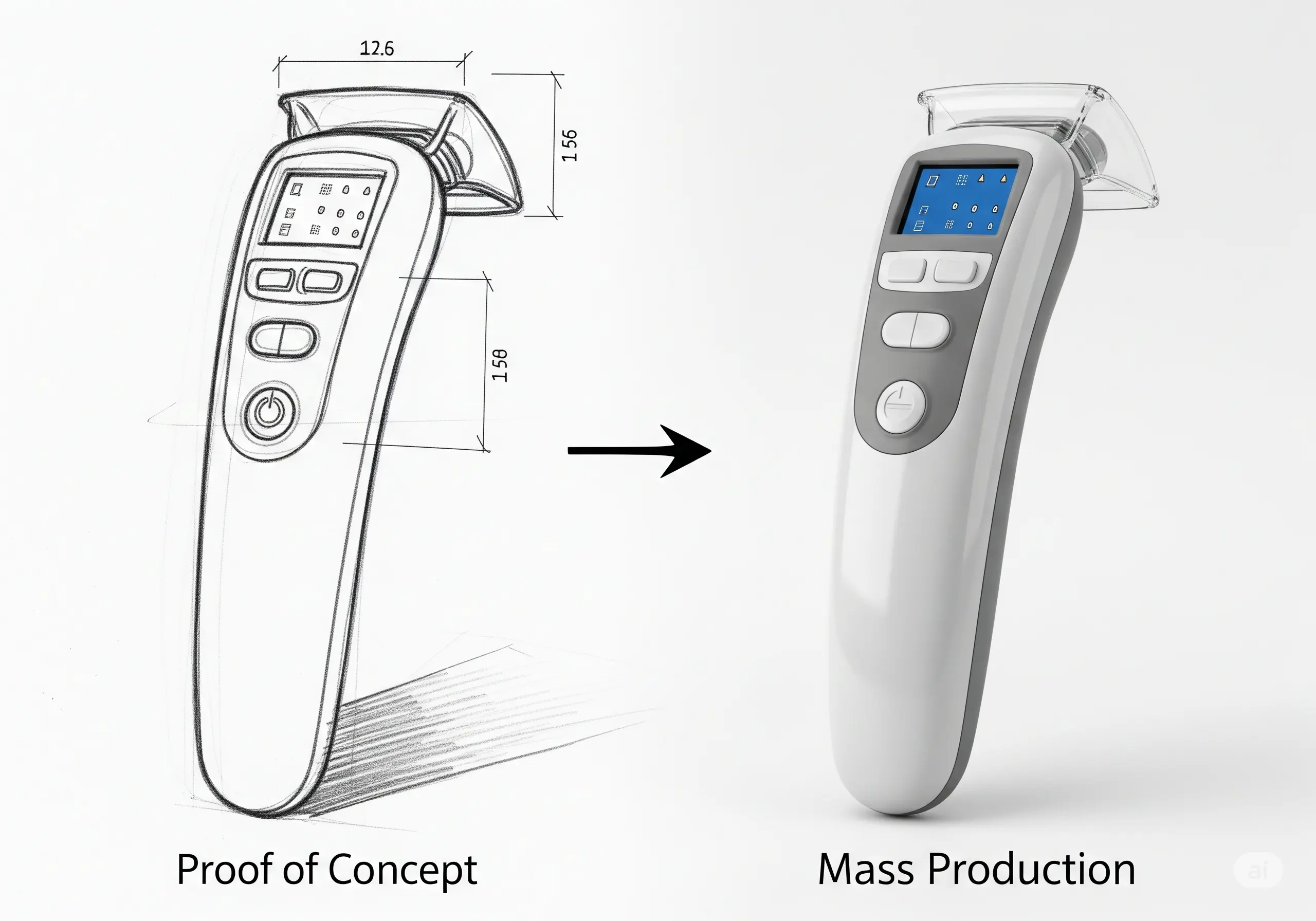

Developing a hardware product, especially in MedTech and Digital Health, involves multiple structured phases, each with distinct goals and challenges. From early Proof of Concept (PoC) to mass production, these stages ensure the product is technically feasible, meets user needs, and complies with regulatory requirements.

1. Proof of Concept (PoC): Validating the Core Idea
The PoC stage is about demonstrating that the core technology or concept works. It’s typically a quick and basic experiment using off-the-shelf components, breadboards, or simulation tools.
- Goal: Validate the fundamental feasibility of the idea.
- Example: Testing an ECG signal acquisition circuit on a breadboard before designing a full-scale wearable ECG device.
- Common Mistakes: Overengineering or focusing too much on aesthetics instead of proving feasibility.
- Solution: Keep it simple—focus on core functions and get early feedback from stakeholders.
2. Prototype: Functional Early Version for Testing
A prototype is the first working model of the product, integrating key functionalities. It is often manually assembled using 3D-printed enclosures, hand-soldered PCBs, or development kits.
- Goal: Test core functionality, not manufacturability.
- Example: A wearable heart rate monitor with 3D-printed housing and an Arduino-based microcontroller.
- Common Mistakes: Treating the prototype as a final product, leading to poor scalability.
- Solution: Use rapid prototyping techniques and expect to refine the design multiple times.
3. Alpha Prototype: Early Testing with Limited Features
The Alpha stage includes a more refined version of the prototype with closer-to-final form factor and materials. It's mainly used for internal testing and validation.
- Goal: Identify design flaws and refine hardware/software.
- Example: A wearable ECG patch with a custom PCB, rechargeable battery, and preliminary firmware.
- Common Mistakes: Ignoring compliance testing at this stage, leading to design rework later.
- Solution: Begin EMC/EMI pre-compliance testing early to avoid costly revisions.
4. Beta Prototype: Near-Final Testing Version
The Beta prototype is close to the final product in form, fit, and function. It is produced in small batches for real-world testing.
- Goal: Conduct usability studies and get feedback from real users.
- Example: A remote patient monitoring device tested in a hospital for usability and performance.
- Common Mistakes: Skipping field testing, which can lead to usability or durability issues.
- Solution: Perform pilot studies and collect real-world feedback.
5. Design Validation Testing (DVT): Finalizing the Design for Manufacturing
DVT is a critical phase where the product undergoes full regulatory and compliance testing (e.g., ISO 13485, IEC 60601, FCC, CE).
- Goal: Ensure the product meets all performance, safety, and regulatory requirements.
- Example: Conducting biocompatibility testing (ISO 10993) for a skin-contacting wearable.
- Common Mistakes: Rushing through this phase, leading to compliance failures during certification.
- Solution: Work with regulatory consultants and test in certified labs early.
6. Design for Manufacturability (DFM): Preparing for Mass Production
DFM focuses on optimizing the design for large-scale manufacturing while maintaining quality and cost efficiency.
- Goal: Ensure the design is scalable, cost-effective, and reliable.
- Example: Refining the plastic enclosure mold design to reduce injection molding defects.
- Common Mistakes: Ignoring component sourcing, leading to supply chain issues.
- Solution: Work closely with contract manufacturers (CMs) to select widely available components.
7. Pilot Production: Small Batch for Final Validation
Pilot production is the first small-scale manufacturing run (e.g., 100–1000 units) to test manufacturing consistency.
- Goal: Identify and fix assembly, quality, and yield issues before full-scale production.
- Example: Detecting a high failure rate in soldering joints on an ECG patch PCB.
- Common Mistakes: Skipping quality control steps, leading to high defect rates in mass production.
- Solution: Implement Manufacturing Process Instructions (MPIs) to standardize assembly.
8. Mass Production: Full-Scale Manufacturing
Mass production is the final stage where large quantities (e.g., 10,000+ units) are produced with consistent quality and cost efficiency.
- Goal: Ensure high-yield, defect-free production.
- Example: Manufacturing 50,000 smart glucometers with automated quality checks.
- Common Mistakes: Underestimating logistics, leading to delays in product launch.
- Solution: Establish robust supply chain management and perform continuous quality control (QC) checks.
Conclusion
Bringing a MedTech or IoT device from concept to market requires structured development phases. Each stage—PoC, prototype, Alpha, Beta, DVT, DFM, pilot, and mass production—serves a critical purpose in ensuring functionality, manufacturability, and compliance.
At ITR VN, we help MedTech startups navigate these stages by providing engineering expertise, regulatory guidance, and contract manufacturer management. Need support? Contact us today!
ITR – A trusted tech hub in MedTech and Digital Health





